BENGALURU: Two research proposals from Karnataka to study the
potential use of convalescent plasma therapy in treating coronavirus
infection are awaiting approval of the Drug Controller General of India
(DCGI). One was sent three weeks ago by a team of doctors attached to a
private hospital in Bengaluru. The other was forwarded as a collective
request recently by dedicated Covid-19 government hospitals.
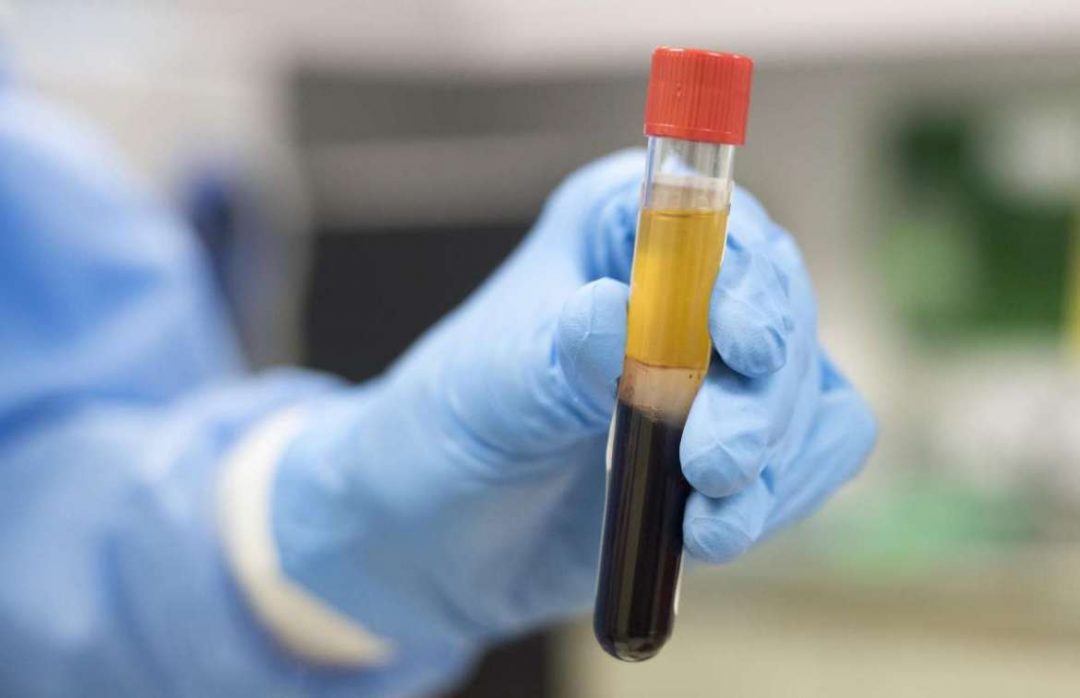
Plasma is the almost clear liquid part of the blood. (About 55% of our
blood is plasma.) The plasma of somebody who has overcome an illness
contains antibodies, protective proteins that fight disease and antigens
like viruses. This is called convalescent plasma.
Globally, the hope is that a transfusion of convalescent plasma, derived from a person who has fully recovered from
coronavirus, could boost the ability of critically ill Covid-19 patients to fight the virus. The therapy is being tested in the US,
China, South Korea, Canada and some other countries. To date, there is no definitive research on its effectiveness in tackling
coronavirus or on any drawbacks. There are some encouraging signs, which is why countries are examining its use.
Early movers
The said approach is being tested in Delhi. In Mumbai, a person who recovered from Covid-19 has donated blood at BYL Nair
Hospital.
Doctors from the immunobiology wing of HCG Cancer Centre, Bengaluru, have sought DCGI’s approval to start experimental
use of the therapy. “There are three categories of Covid-19 patients: those who are asymptomatic, those (2A) who are
vulnerable for further disease progression and those (2B) with significant symptoms, and people battling severe comorbidities
like respiratory distress. It’s the third category which needs convalescent plasma therapy,” said Dr Vishal Rao US, associate
dean, Centre of Academics & Research, HCG Cancer Centre. “We have moved a research application to DCGI and ICMR [Indian
Council of Medical Research] parallelly, seeking permission to start clinical trials with the Karnataka government.”
A fee of Rs 3 lakh has to be paid while filing a research application with DCGI. “A research institute or a hospital must seek
approval for conducting studies and clinical trials on plasma therapy on its own, and the state governments hardly have a role
to play here,” said a senior researcher from Bengaluru, adding that DGCI should review the applications quickly considering the
severity of the crisis.
Doctors at dedicated Covid-19 government hospitals have also been discussing the potential use of plasma therapy. “In some
countries, plasma therapy is said to have helped severely ill Covid-19 patients. We have written to the Bangalore Medical
College and Research Institute,” said Dr C Nagaraja, director, Rajiv Gandhi institute of Chest Diseases, the first nodal centre in
Karnataka for treating Covid-19 patients.
BMCRI has sent a research request on behalf of Covid-19 hospitals in Bengaluru to central agencies. “The ethical committee of
BMCRI has approved it. It was sent to the medical education department for further forwarding to DCGI,” said Dr CR Jayanthi,
dean and director, BMCRI.
Jawaid Akhtar, additional chief secretary, state health and family welfare department, confirmed some government institutions
had sought permission for plasma therapy research. “We have forwarded the requisition to DCGI and ICMR,” he said.